Recently, various approaches to improve analytical SEC have focused on reducing the analysis time. This can be achieved by staggered injection protocols or increased linear flow rates. The mobile phase composition itself leaves less room for method improvement, compared to other chromatographic modes. As soon as a certain ionic strength (inhibiting electrostatic interactions without causing hydrophobic interactions) and the pH of the mobile phase (ensuring structural integrity of proteins and the stationary phase) are set, one might think that the analysis solely depends on the particle size, packing quality and column length. However, the mobile phase composition is not at the end of its rope, in case the mentioned parameters have been set. For example, arginine and other amino acids can be added to the mobile phase to affect the aggregate recovery in SEC.
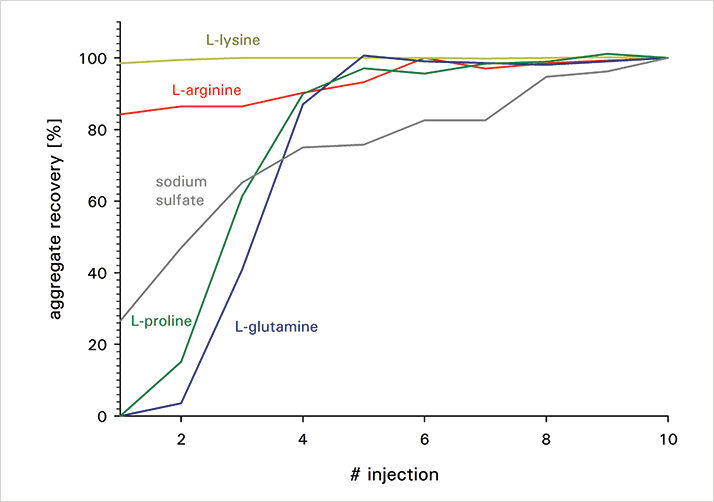
A mAb was aggregated by incubation at 75 °C for 5 min. Subsequently, the sample was analyzed via TSKgel UltraSW Aggregate 7.8 mm ID x 30 cm L with different mobile phases, all of them using virgin columns. 0.2 M lysine, arginine, proline, glutamine or sodium sulfate were added to 0.1 M sodium phosphate buffer, pH 6.7, respectively. A flow rate of 1 mL/min was applied, and 20 µl (100 µg) of the aggregated mAb samples were injected onto equilibrated columns. Figure 1 illustrates the results on aggregate recovery. Glutamine and proline show a similar behavior: the aggregates are hardly recovered for the first two injections, while the aggregate peak suddenly appears for injection #3 and #4. The rise is not as sudden for sodium sulfate, but the aggregate peak will only achieve its full size for injection #10. In opposition to these results, lysine shows an even improved aggregate recovery compared to arginine. The inter-injection variability is low, depicting the complete aggregate content for all of the injections.
Besides aggregate recovery, resolution of the different sample components, namely the monomer and the different aggregates, is crucial for accurate analysis. Clearly, there is motivation to increase resolution. Figure 2 depicts the separation profile of an aggregated mAb sample on TSKgel UltraSW Aggregate using 0.1 M sodium phosphate buffer, pH 6.7 with an addition of 0.2 M arginine. Ten injections with the arginine buffer were followed by ten injections applying sodium phosphate buffer with an addition of 0.2 M sodium sulfate, in order to compare the two buffers. Monomer aggregate resolution, as well as monomer fragment resolution is slightly improved for the amino acid buffer.
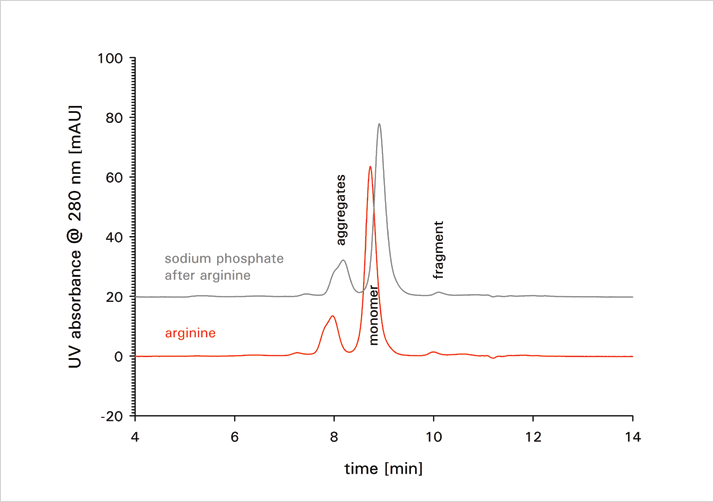
Besides arginine, both proline and glutamine provide slightly increased monomer aggregate resolution. For arginine, the fragment monomer resolution is also improved. Although these increases in resolution are not drastic, they confirm that increased resolution due to the use of an advanced mobile phase is possible and that mobile phase testing can contribute to a more reliable and robust aggregate analysis.
